anti-sars-cov-2 igg elisa kit|SARS : China Sensitivity and specificity of four serum-based ELISAs: Detection of IgG or IgA antibodies against SARS-CoV-2 in serum samples collected prior to the spread of COVID-19 (pre-Nov. 2019), and people . Cover with tin foil and use autoclave tape to secure it to the flask. Place flask in the "To Be Autoclaved" section. As the agar will begin cooling as soon as it is in the autoclave, ask Nicole to put the flask in the waterbath at 55C so that the solution stays liquid.
{plog:ftitle_list}
Two commonly used sterilization methods are pressure cookers and autoclaves. While both methods utilize heat and pressure to eliminate microorganisms, they differ .
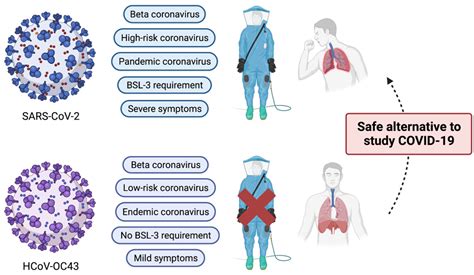
About This Kit. The Human SARS-CoV-2 Spike (Trimer) IgG ELISA is a serology assay that measures and quantitates immunoglobulin G (IgG) antibodies against SARS-CoV-2 Spike .The EUROIMMUN Anti- SARS-CoV-2 ELISA (IgG) is an enzymelinked immunosorbent assay intended - for the qualitative detection of IgG class antibodies to SARS- CoV-2 in human .SARS-CoV-2 IgG ELISA Kit (ab275300) is an Enzyme-Linked Immunosorbent Assay (ELISA) intended for semi-quantitative detection of IgG antibodies to SARS-CoV-2 in human serum or .This product is used for in vitro quantitative detection of anti-SARS-CoV-2 IgG antibodies in mouse serum, plasma and other culture samples. It can help assess the amount of IgG antibodies produced by mouse after immunization or mouse IgG antibodies expressed in cell culture.
Sensitivity and specificity of four serum-based ELISAs: Detection of IgG or IgA antibodies against SARS-CoV-2 in serum samples collected prior to the spread of COVID-19 (pre-Nov. 2019), and people .
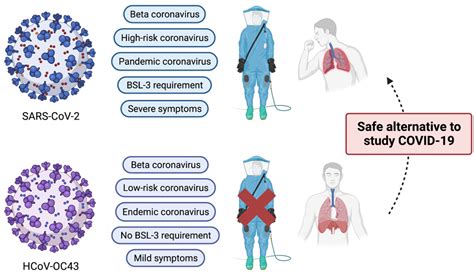
SARS-CoV-2 N-Protein IgG Antibody ELISA Kit for Measurement of Human SARS-CoV-2 N-Protein IgG Antibody in Plasma, Serum etc. English +1 877 302 8632; Contact . Real quantitative determination of absolute anti-SARS-CoV-2 Nucleocapsid IgG antibodies Sample Type Plasma, Serum . At a 1:100 dilution of serum in the SARS-CoV-2 spike ELISA, . in small-scale test cohort for SARS-CoV-2 IgG, . resolution profiling of anti-SARS-CoV-2 antibodies for detecting early .
SARS-CoV-2 IgG ELISA Kit (ab275300) is an Enzyme-Linked Immunosorbent Assay (ELISA) intended for semi-quantitative detection of IgG antibodies to SARS-CoV-2 in human serum or plasma collected in potassium EDTA, sodium citrate or lithium heparin. . is proportional to the amount of RBD-specific anti-SARS-CoV-2 IgG present in the sample.Objectives: To evaluate and compare the performances of five commercial ELISA assays (EDI, AnshLabs, Dia.Pro, NovaTec, and Lionex) for detecting anti-SARS-CoV-2 IgG. Methods: Seventy negative control samples (collected before the COVID-19 pandemic) and samples from 101 RT-PCR-confirmed SARS-CoV-2 patients (collected at different time points from symptom onset: . Methods. We compared the performance of three immunoassays, an in-house RBD assay, and two commercial assays, the Diasorin LIAISON SARS-CoV-2 S1/S1 IgG CLIA which detects antibodies against S1/S2 domains of the Spike protein and the Zydus Kavach assay based on inactivated virus using a well-characterized panel of sera. 379 sera and plasma .SARS-CoV-2 / COVID-19 ELISA kits for research setting. Quantitative and qualitative IgG/IgM ELISA kits to estimate antibodies in samples of COVID-19 patients. . Our SARS-CoV-2 sandwich ELISA immunoglobulin kits have been extensively tested against anti-SARS-CoV-2 verification panel for serology assays 20/B770 from the National Institute for .
SARS
In order to address this challenge, the present study evaluated the performance of five commercial CE-marked ELISA kits for detecting anti-SARS-CoV-2 IgG antibodies in samples from RT-PCR-confirmed COVID-19 patients. The sensitivity, positive predictive value, negative predictive value, positive percent agreement, and Cohen’s kappa were . We used the commercial Euroimmun ELISA for the determination of anti-N IgM, the Abbott Architect ™ SARS-CoV-2 IgG test for the detection of anti-RBD IgG, and an in-house kit for the assay of anti-N IgG and anti-N IgA. IgM and IgA antibodies were assessed 2–5, 9–12, 17–20 and 32–37 days after symptom onset.The SARS-CoV-2 Neutralizing Ab ELISA kit is designed to measure the neutralizing portion of anti-SARS-CoV-2 antibodies. A receptor binding domain (RBD) protein is pre-coated in the wells of the supplied microplate. Samples or the positive control are added into the wells.
SARS-CoV-2 Spike Protein IgG ELISA Kit (DEIASL064), manufactured by Creative Diagnostics with high sensitivity and reproducibility, providing efficient and accurate result for lab research. . This immunoassay kit allows for the qualitative determination of anti-SARS-CoV-2(S)-IgG in human serum, plasma, saliva and nasal fluid. ELISA was performed with an IDK® anti-SARS-CoV-2 IgG ELISA kit from Immundiagnostik AG (Germany) strictly following the provided protocol. Pre-coated and blocked 96-well plates were incubated .SARS-CoV-2 IgG Quantitative ELISA Kit (DEIASL019Q), manufactured by Creative Diagnostics with high sensitivity and reproducibility, providing efficient and accurate result for lab research. . Human Anti-SARS-CoV-2 N+S-RBD Monoclonal antibody, clone COTHN12. Rabbit Anti-SARS-CoV-2 RBD Monoclonal Antibody, clone 28C. Correlation with anti-SARS-CoV-2 binding antibodies was variable, but the strongest correlations were observed between anti-RBD IgG antibodies and Mindray (r = 0.952, R2 = 0.907). . . Evaluation .
SARS-CoV-2 S1RBD IgG ELISA Kit is an immunoassay to quantify IgG antibodies against S1RBD of SARS-CoV-2 and determine COVID-19 vaccine efficacy. It has been calibrated with WHO International Standard (NIBSC Code: 20/136), and can be used to test the effectiveness of COVID-19 vaccines. . 1 x 12 ml Stop Solution: 1 x 12 ml 10x Standard Solution .
The stabilized anti-RBD IgG ELISA kit was evaluated for its performance by testing large sera panels from SARS-CoV-2 RT-PCR positive individuals . (Covid Kavach IgG) from Zydus diagnostics, India and Anti-SARS-CoV-2 ELISA (IgG) from Euroimmun, Germany. In the Zydus ELISA the wells are coated with inactivated SARS-CoV-2. Whereas, in Euroimmun .
Buy SARS-COV-2 Spike RBD Protein IgG Antibody ELISA Kit, item number: G-CBK4137.96 from Assay Genie at Biomol! Protein function: [Spike protein S1]: attaches the virion to the cell membrane by interacting with host receptor, initiating the infection.SARS-CoV-2 Rapid Antigen Test 2.0 Nasal The SARS‑CoV‑2 Rapid Antigen Test 2.0 Nasal is a rapid chromatographic immunoassay for the qualitative detection of SARS‑CoV‑2 nucleocapsid antigen present in human nasal samples. This test is intended as an aid in the diagnosis of SARS‑CoV‑2 infection in individuals with or without symptoms consistent with COVID‑19.This .
how to tell when fermentation is complete conical refractometer
Serum samples were processed and analyzed for SARS-CoV-2 IgG antibodies and anti N-protein IgG levels using an ELISA kit for IgG by Epitope Diagnostics 16 according to manufacturer’s protocol .IgG 1vial Diluteat1:100with1×dilutionbufferfor 10minutesbeforeuse.Dilutefreshas needed. . reconstituted,The workingfluidisused withintheworking dayanddiscard.So dilutefreshas needed. Anti-SARS-CoV-2 (2019-nCoV)Spike RBD(Control) 1vial Gradientdilutedat1:80000-1:320000 with1×dilutionbufferfor10minutes . SARS-CoV-2 (2019-nCoV) Spike ELISA KitIn order to address this challenge, the present study evaluated the performance of five commercial CE-marked ELISA kits for detecting anti-SARS-CoV-2 IgG antibodies in samples from RT-PCR-confirmed COVID-19 patients. The sensitivity, positive predictive value, negative predictive value, positive percent agreement, and Cohen’s kappa were .
how to test a refractometer
Background An assessment of the factors that interfere with serum levels and the persistence of anti-SARs-CoV-2 IgG antibodies is essential in order to estimate the risk of reinfection and to plan vaccination. We analyzed the impact of the severity of coronavirus disease 2019 (COVID-19) and the clinical and biological factors regarding the persistence of SARs .SARS-CoV-2 Nucleocapsid IgG Quantitative ELISA Kit (DEIASL062), manufactured by Creative Diagnostics with high sensitivity and reproducibility, providing efficient and accurate result for lab research. . This kit is intended for quantitative detection of Anti-SARS-CoV-2(N) IgG in serum, plasma and other biological fluids.This product is used for in vitro quantitative detection of anti-SARS-CoV-2 IgG1 antibodies in mouse serum, plasma and other culture samples. It can help assess the amount of IgG1 antibodies produced by mouse after immunization or mouse IgG1 antibodies expressed in .
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has triggered the worldwide coronavirus disease 2019 (COVID-19) pandemic. Serological assays for the detection of SARS-CoV-2 infections are important to understand the immune response in patients and to obtain epidemiological data about the number of infected people, especially to identify .The Rat Anti-SARS-CoV-2 (Covid-19) IgG Antibody to Nucleoprotein Quantitative TITRATION ELISA kit is used as an analytical tool for quantitative estimation of Anti-SARS-CoV-2 (2019-nCoV) Nucleoprotein antibodies in rat serum.
Human SARS
Anti
$439.00
anti-sars-cov-2 igg elisa kit|SARS